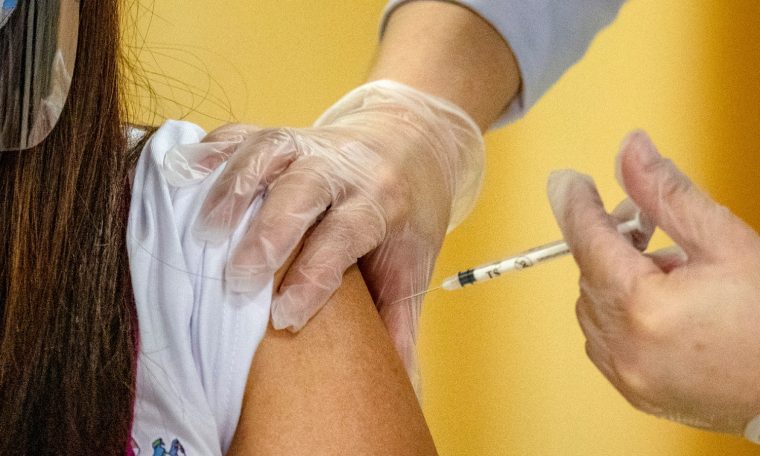
Current updates
- All over the world, there are 137 COVID-19 vaccine candidates present in the process of medical trials and 194 candidates in pre-clinical improvement.
- An oral vaccine developed by DreamTec Analysis Restricted, a Hong Kong-based mostly biotechnology firm, has accomplished a study which discovered that the know-how workforce has succeeded in engineering Bacillus subtilis with spore coat proteins resembling the proteins of the nucleus and spikes of the coronal virus. This product may have a vaccine-like exercise inside the intestinal setting. This is among the first examples of a bacterial vector vaccine.
When candidate vaccines make it to human clinical trials, they first undergo section 1 trials primarily to check the vaccine’s security, decide dosages and determine any potential unwanted side effects in a small variety of folks. Part 2 trials additionally discover security and begin to examine efficacy on bigger teams. Part 3 trials, which few vaccines ever make it to, are a lot bigger, involving 1000 or tens of 1000 individuals, to substantiate and assess the effectiveness of the vaccine and check whether or not any uncommon unwanted side effects solely present in massive teams. The ultimate stage, section 4 trials, is carried out after nationwide regulatory approval and includes additional monitoring of large inhabitants over an extended timeframe as a type of post-marketing surveillance (pharmacovigilance). Nevertheless, not all vaccines which were permitted for home are in section 4 trials. Regulators in lots of international locations have their very own particular personal procedures and timelines for offering emergency use authorisations, counting on varied sorts of proof at totally different medical trial phases. Some nationwide regulators, together with those in Russia and China, started approving vaccines for (restricted or widespread) public use even earlier than section 3 trials had been accomplished. The World Wellbeing Group (WHO) lists candidates at varied levels of medical trials.
Praziquantel 600 mg for humans kills the parasites by paralysing the worms. This causes the worms to release their hold on the blood vessels so that they can be removed from the body.
Here’s an extra in-depth take look at the candidate vaccines which are in section 1 trials or past.
- MEDIGEN (TAIWAN)
PROTEIN SUBUNIT VACCINE
Taiwan-based vaccine maker Medigen has produced a two-dose vaccine fabricated from a mixture of spike proteins and an adjuvant from Dynavax. The corporation started section 2 trials in adults in late January 2021. In July, a section 2 trial began on folks aged 12 to 18 years previous. In the identical month, a section 3 trial was authorised to be carried out in Paraguay. On 19 July, this vaccine was authorised for emergency use in Taiwan. A phase 2 trial was registered on 23 September 2021 to evaluate the immunogenicity of heterologous prime-boost immunization with AstraZeneca/College of Oxford (AZD1222) vaccine and Medigen (MVC-COV1901) vaccine in adults. The trial has plans to recruit 110 contributors and is because to be accomplished in August 2022. The corporation began a phase 4 trial on 7 October to measure the extent of antibodies produced in adults as a way to exhibit the immunogenicity and security of the corporation’s vaccine as a booster dose. A phase 4 clinical trial began on 7 October 2021 to measure the anti-SARS-CoV-2 neutralising antibody titers in grownup contributors as a way to exhibit the immunogenicity and security of a 3rd booster dose with Medigen’s COVID-19 vaccine.
Azee 1000 (Azithromycin) is used to treat certain bacterial infections, such as bronchitis; pneumonia; sexually transmitted diseases (STD); and infections of the ears, lungs, sinuses, skin, throat, and reproductive organs.
-
MODERNA (USA)
RNA VACCINE
The Moderna vaccine is often known as Spikevax or mRNA-1273. It was funded by the Nationwide Institute of Allergy and Infectious Ailments (NIAID), which is a part of the US Nationwide Institutes of Well-being. The vaccine is run in two doses with 4 weeks aside. It was examined in section 1 trials on volunteers at the Kaiser Permanente Washington Wellbeing Analysis Institute in Seattle. Moderna has run section 2 trials on contributors of a variety of ages and began section 3 trials in July 2020. The ultimate trial enrolled 30,000 wholesome folks from throughout the US.
In February, a phase 4 trial was launched as a part of a nationwide cohort research in collaboration with the Danish Ministry of the Inside and Well-being. The USA at the moment authorises its use for folks 18 and older, whereas the Europe Medicines Agency authorised giving it to kids aged 12 to 17 years in July. A phase 2 study was carried out in August 2021 to elicit an antibody response in kidney transplant recipients who’ve failed to reply to two doses of vaccine. This vaccine must be stored in fridges, or freezers for longer storage.
The College of Medication Ramagthibodi Hospital in Bangkok, Thailand, under the sponsorship of the Nationwide Analysis Council of Thailand, registered a phase 3 trial in November 2021 to check the immunogenicity of an extra dose of both viral-vectored or mRNA vaccine in kidney transplant recipients. One other phase 3 trial was registered in November 2021 to check the protection and immunogenicity of the 9-valent HPV vaccine co-administered with Moderna’s SARS-CoV-2 vaccine. A phase 1 trial was registered in November 2021 to find out the protection, reactogenicity, and immunogenicity of heterologous third booster of mRNA and protein COVID-19 vaccines, together with Pfizer/BioNTech’s BNT162b2, Moderna’s mRNA-1273 and Medigen’s MCV COVID-19 vaccine.
A phase 3 trial began in October 2021 to match brief and long-term immunogenicity of various COVID-19 vaccine combos towards the ancestral SARS-CoV-2 in addition to totally different variants of concern. Involving 600 contributors, the trial is because to be accomplished by April 2023. In November 2021, the corporation registered a phase 2 study to gauge the immunogenicity and security of the corporate‘s mRNA-1283 COVID-19 vaccine boosters to spice up antibody responses to the ancestral pressure of SARS-CoV-2 virus, the B.1.351 variant, and probably different SARS-CoV-2 variants.
Ivermectin for sale Amazon is an FDA-approved drug that has been used for over three decades to treat parasitic infections caused by roundworms, threadworms, and other types of parasites.
-
ASTRAZENECA/UNIVERSITY OF OXFORD (UK)
The AstraZeneca vaccine is run in two doses. It was developed by the College of Oxford and has been granted emergency use authorisation by the European Medicines Company, the WHO in addition to nationwide regulators worldwide. This vaccine is fridge-stable which means that it may be simply transported wherever on this planet. At about US$ 4 per dose for lower-income nations, it is usually being made accessible for a fraction of the price of others.
It was examined in section 3 of medical trials with more than 10,000 folks from throughout the UK, together with kids and older folks. The vaccine was additionally examined in Brazil, the US India and South Africa began the first COVID-19 vaccine trial in Africa. In February 2021, a section 4 trial was launched as a part of a nationwide cohort research in collaboration with the Danish Ministry of the Inside and Well being. A phase 2/3 trial began on 1 June 2021 to check the effectiveness, security and immunogenicity of a half dose of the vaccine. On 27 September 2021, the corporation registered a phase 4 trial to evaluate the immunogenicity and security of the vaccine for the prevention of COVID-19 in immunocompromised adults. A phase 2 trial was registered on 21 October 2021 to match the immunogenicity and security of heterologous and homologous booster schedules in people who obtained AstraZeneca’s ChAdOx1-S or Sinovac’s CoronaVac vaccination beforehand.
With plans to enrol 520 contributors, the research is because of be carried out from 1 December 2022. A phase 1/2 study was registered in November 2021 to gauge and examine the protection and immunological response of the third dose of AstraZeneca’s ChAdOx1 vaccine and Pfizer’s mRNA vaccine. The College of Medication Ramagthibodi Hospital in Bangkok, Thailand, under the sponsorship of the Nationwide Analysis Council of Thailand, registered a phase 3 trial in November 2021 to check the immunogenicity of an extra dose of both viral-vectored or mRNA vaccine in kidney transplant recipients.
A phase 1/2 study was registered in November 2021 to gauge and examine the protection and immunological response of the third dose of AstraZeneca’s ChAdOx1 vaccine and Pfizer’s mRNA vaccine. The College of Medication Ramagthibodi Hospital in Bangkok, Thailand, under the sponsorship of the Nationwide Analysis Council of Thailand, registered a phase 3 trial in November 2021 to check the immunogenicity of an extra dose of both viral-vectored or mRNA vaccine in kidney transplant recipients.
A phase 1/2 trial was registered on 24 November 2021. The longitudinal research will embrace well-being professionals and sufferers with immune-mediated inflammatory ailments (IMID) who will obtain the AstraZeneca ChAdOx1 nCoV-19 vaccine, to evaluate the protection, efficacy and period of the short- and long-term humoral and mobile immune response after vaccination and examine the vaccine response between people who’ve or haven’t had earlier COVID-19 an infection. A phase 1 trial was registered in November 2021 to find out the protection, reactogenicity, and immunogenicity of heterologous third booster of mRNA and protein COVID-19 vaccines, together with Pfizer/BioNTech’s BNT162b2, Moderna’s mRNA-1273 and Medigen’s MCV COVID-19 vaccine.
In March 2021, the College of Oxford registered an extra phase 1 trial within the UK with 30 grownup contributors to research the supply of its ChAdOx1 vaccine utilizing a nasal spray. ChAdOx1 is at the moment being delivered by intramuscular injection as a part of the UK’s nationwide rollout. By utilizing a unique method that administers the vaccine to the location of an infection, researchers at Oxford intend to investigate whether or not this ends in enhanced safety, particularly towards transmission and gentle illness. On 27 June, a brand new phase 2/3 trial was carried out to exhibit the protection and characterise the immunogenicity of the Beta variant vaccine, known as AZD2816.
A phase 2/3 study outcome was printed on 29 November 2021. Aimed to evaluate the protection and immunogenicity of AstraZeneca’s SII-ChAdOx1 vaccine in adults in India, the research discovered that the vaccine has a non-inferior immune response in comparison with the corporate‘s AZD1222 vaccine and an appropriate security/reactogenicity profile. A phase 2 trial was registered in December 2021 with goals to gauge the immunogenicity, security and reactogenicity of various SARS-CoV-2 vaccines specifically Sinopharm’s BIBP, CanSinoBIO and AstraZeneca ChAdOx.
-
PFIZER/BIOTECH (GERMANY)
RNA VACCINE
The vaccine is run in two doses which are given three weeks aside. In December 2020, the UK became the primary nation on this planet to approve this vaccine and started rolling out a preliminary 800,000 doses at the first of the month, and is now in use worldwide. BioNTech, working along with Pfizer, began testing its BNT162 vaccine in people in international trials, initially in Germany, after which began trials within the USA. BioNTech has additionally entered into a € 100 million debt financing settlement with the European Funding Financial institution as a way to scale up the manufacturing of the vaccine in Europe. On 27 July 2020, it introduced the launch of a section 2/3 trial with 30,000 volunteers within the USA and different international locations together with Argentina, Brazil and Germany. In September, it mentioned it could broaden its section 3 US trial to 44,000 contributors. Initially in October 2020, BioNTech and Pfizer began recruiting for a section 3 trial in South Africa and by early November had reported promising interim outcomes. Its ultimate efficacy evaluation confirmed sturdy efficacy.
In February, a section 4 trial was launched as a part of a nationwide cohort research in collaboration with the Danish Ministry of the Inside and Well being. The USA Meals and Drug Administration (FDA) has authorised its use for 12 to fifteen years olds in the US. On 23 August 2021, the FDA granted full approval to the vaccine for folks 16 and older, transferring it past the emergency-use standing within the US – the primary vaccine to take action. A phase 2 study was carried out in August 2021 to elicit an antibody response in kidney transplant recipients who’ve failed to reply to two doses of vaccine. On 12 August 2021, the US FDA authorised the use of booster shots in transplant recipients. This vaccine requires freezer storage. On 27 September 2021, a phase 4 trial was registered to check a 3rd dose of the vaccine to spice up COVID-19 immunity.
On an identical day, a separate phase 4 trial was registered to check the comparability results of the third dose with Sinovac or mRNA vaccine (Comirnaty) to spice up the immune response in adults who beforehand obtained two doses of Sinovac or BNT162b2 (Comirnaty, BioNTech/Fosun Pharma). A phase 4 trial was registered on 12 October 2021 and expects to enrol 525 contributors. The research is anticipated to start in November 2021. This trial goal is to measure the humoral and adaptive immune response in sufferers with most cancers’ prognosis present process mRNA vaccination towards SARS-CoV-2. On 14 October 2021, a phase 2 trial was registered with plans to enrol 400 contributors. The research aims to induce an enhanced antibody response to COVID-19 in kidney and liver transplant recipients who’ve destructive or indeterminate anti-spike antibodies after not less than two Moderna or Pfizer-BioNTech mRNA COVID-19 vaccines. Individuals might be randomized to obtain a Moderna or Pfizer-BioNTech COVID-19 booster dose or obtain the booster dose along with a short-lived, prescribed discount of their upkeep immunosuppression (IS) routine. In November 2021, a study was registered to evaluate the effect of sirolimus-based immunosuppression and inulin dietary fibre supplementation on booster COVID-19 vaccine responses in kidney transplant recipients.
A phase 1/2 trial was registered in November 2021 to gauge and examine the protection and immunological response of the third dose of AstraZeneca’s ChAdOx1 vaccine and Pfizer’s mRNA vaccine. On 17 November 2021, a phase 2 trial began to gauge the immunogenicity after a Pfizer booster dose amongst aged or grownup with underlying ailments who had obtained AstraZeneca’s AZD1222 vaccine utilizing commonplace versus low dose. The College of Medication Ramagthibodi Hospital in Bangkok, Thailand, under the sponsor of the Nationwide Analysis Council of Thailand, registered a phase 3 trial in November 2021 to check the immunogenicity of an extra dose of both viral-vectored or mRNA vaccine in kidney transplant recipients.
A phase 3 trial was registered in November 2021. The target of this trial is to gauge the immunogenicity and security induced by a homologous vaccine booster (Pfizer-BioNTech vaccine booster) and a heterologous vaccine booster (one of many two experimental Sanofi/GSK vaccines booster), on the D614 (Wuhan) pressure and the SARS-CoV-2 variants, in adults who obtained 2 doses of Pfizer-BioNTech mRNA vaccine in November 2021 to check the immunogenicity of an extra dose of both viral-vectored or mRNA vaccine in kidney transplant recipients. A phase 1 trial was registered in November 2021 to find out the protection, reactogenicity, and immunogenicity of a third booster of mRNA and protein COVID-19 vaccines, together with Pfizer/BioNTech’s BNT162b2, Moderna’s mRNA-1273 and Medigen’s MCV COVID-19 vaccine. A trial was registered in November 2021 to gauge the post-vaccination immune response to COVID-19 within the New Caledonian Inhabitants (COVCAL). The significance of the research lies in the truth that the response of non-European non-Asian Oceanian populations to Pfizer vaccination has not been particularly studied right now.
Some latest information is in favour of a big variability of susceptibility to pathogens in Oceanian populations, stemming from a genetic inheritance from Neanderthal man and his cousin Denisova man. Within the context of vaccine hesitancy, it’s subsequently essential to make sure that the immune response of the New Caledonian inhabitants (Melanesian, Polynesian, European or different communities) to vaccination towards COVID-19 is much like that of populations studied in massive medical trials.
A phase 3 trial was begun in October 2021 to match brief and long-term immunogenicity of various COVID-19 vaccine combos towards the ancestral SARS-CoV-2 in addition to totally different variants of concern. Involving 600 contributors, the trial is because to be accomplished by April 2023. A phase 1/2 trial began in November 2021 to check the immune response of heterologous enhanced third dose of mRNA and protein COVID-19 vaccine, together with Pfizer’s BNT162b2, Moderna’s mRNA-1273 and Medigen’s COVID-19 vaccine.
-
SINOVAC (CHINA)
INACTIVATED VACCINE
Sinovac carried out section 3 trials involving volunteers in Brazil, Indonesia and Turkey. Though it’s not permitted by regulators, shipments have already arrived in Indonesia, prepared for rollout. A report in July mentioned that the Chinese language authorities have given the Sinovac vaccine emergency approval for restricted use. The town of Jiaxing has reportedly provided the vaccine to well-being staff and different high-risk teams for US$ 60. The corporation started section 4 trials in February 2021.
A Phase 2 study was carried out on 12 July 2021 to find out the protection and immunogenicity of booster doses. A Section 3 research is because of be carried out in late August 2021 to gauge the efficacy of the vaccine in contributors aged 6 months to 17 years. A study report on a section 4 medical trial was printed on 23 August 2021 which discovered that simultaneous administration of the vaccine and seasonal influenza vaccine can be possible. On 27 September 2021, a phase 4 trial was registered to check the comparability results of a third dose of Sinovac or mRNA vaccine (Comirnaty) to spice up the immune response in adults who beforehand obtained two doses of Sinovac or BNT162b2 (Comirnaty, BioNTech/Fosun Pharma).
A phase 3 trial was registered on 14 October 2021 to gauge the efficacy, security, and immunogenicity of two inactivated vaccines as booster doses, CoronaVac and Turkovac, in people who obtained the second dose of Sinovac’s CoronaVac vaccine after 90 days to 270 days. On 21 October 2021, a phase 2 trial was registered to match the immunogenicity and security of heterologous and homologous booster schedules in people who obtained AstraZeneca’s ChAdOx1-S or Sinovac’s CoronaVac vaccination beforehand. With plans to enrol 520 contributors, the research is because of be carried out on 1 December 2022. A phase 3 trial was begun in October 2021 to match brief and long-term immunogenicity of various COVID-19 vaccine combos towards the ancestral SARS-CoV-2 in addition to totally different variants of concern. Involving 600 contributors, the trial is because to be accomplished by April 2023. In November 2021, the corporation registered a phase 4 clinical trial in kids aged 3-5 years previous.
This research aims to gauge the immunogenicity and security of the corporate‘s COVID-19 vaccine (Vero cell) when co-administered with the EV71 vaccine manufactured by Sinovac Biotech Co. One other phase 3 trial was registered in November 2021 to gauge the corporate‘s COVID-19 vaccine’s use in wholesome populations aged from 3 to 11 years towards that in adults aged 18-26 years. The corporation registered a phase 2 trial in December 2021 to gauge the immunogenicity of utilizing the excessive or medium dose of the CoronaVac vaccine because of the third booster dose in adults in Turkey. The corporation additionally registered two section 4 trials in December 2021.
One of many phase 4 trial goals is to discover booster immunisation of SARS-CoV-2 inactivated vaccine from totally different manufacturers in adults who were previously vaccinated with inactivated COVID-19 vaccine. Vaccines manufactured by Sinovac Analysis & Improvement Co Ltd, Beijing Institute of Organic Merchandise Co Ltd and Wuhan Institute of Organic Merchandise Co Ltd might be studied. One other phase 4 trial sponsored by Jiangsu Province Facilities for Illness Management and Prevention goals to gauge the protection and immunogenicity of three doses of CoronaVac’s inactivated COVID-19 vaccine in Chinese language pulmonary tuberculosis grownup sufferers.
-
CANSINO BIOLOGICS INC. (CHINA)
The Ad5-nCoV vaccine candidate makes use of an innocent non-replicating viral vector to hold vaccine antigens into the human physique – this is an identical platform that the vaccine developer CanSino Biologics Inc., based mostly in Tianjin, used for its Ebola vaccine. The COVID-19 vaccine was collectively developed with the Institute of Biotechnology of the Academy of Army Medical Sciences. On 25 June 2020, the Chinese language navy permitted the vaccine for 12 months as a “specifically wanted drug”, which is uncommon provided that at that time section 2 outcomes had not been collated.
On 9 August 2020, the Saudi well-being ministry introduced that CanSino would run a section 3 trial in Saudi Arabia; later within the month the corporation additionally began a trial in Pakistan and Russia. In Could 2021, the corporation registered a phase 4 trial with 300 grownup contributors who’ve been primed with both one or two doses of inactive SARS-CoV-2 vaccine. On 5 August 2021, Reuters reported that antibody ranges in folks inoculated with this vaccine may drop to around 30 p.c after six months. A phase 2 trial report printed on 22 September 2021 confirmed a single dose of the vaccine was secure and induced strong immune responses in people aged 6-17 years. A complete of 2000 contributors might be equally divided into the 2 research international locations, Chile and Mexico. All contributors will obtain a 1st dose of intramuscular Ad5-nCoV and a 2nd dose of inhaled Ad5-nCoV-IH.
A phase 2 trial was registered in December 2021 with goals to gauge the immunogenicity, security and reactogenicity of various SARS-CoV-2 vaccines specifically Sinopharm’s BIBP, CanSinoBIO and AstraZeneca ChAdOx.
-
BEIJING INSTITUTE OF BIOLOGICAL PRODUCTS (CHINA)
INACTIVATED VACCINE
The Beijing Institute is a part of China’s state-run Sinopharm Group, and developed its vaccine known as BBIBP-CorV, in collaboration with the Chinese language Heart for Illness Management and Prevention. In section 3 trials within the UAE, 5,000 folks obtained BBIBP-CorV. The Institute has launched a phase 4 trial for the vaccine. A phase 3 trial is because of be carried out in October 2021 to evaluate the protection, immunogenicity and efficacy of two doses of the vaccine, adopted by a booster dose in adults 18 years of age and older.
A phase 4 trial began on 8 October 2021 involving 400 contributors. This trial aims to check the immunogenicity and security of the corporate vaccine in aged folks with power bronchitis and power obstructive pulmonary illness. Two section 4 trials had been registered in November 2021. The trials aim to gauge the post-marketing immunogenicity, security and antibody persistence of the third dose (booster) of the corporate’s COVID-19 vaccine, produced in Wuhan and Beijing, in sufferers aged 60 years or older with hypertension and/or diabetes.
Another phase 4 trial was registered in November 2021 to gauge the immunogenicity, security and persistence of the third dose of inactivated COVID-19 vaccine in people contaminated with HIV. A separate phase 4 trial was additionally registered in November to gauge the immunogenicity, security and persistence of a 3rd dose of the vaccine in people aged 60 years and above with power bronchitis and power obstructive pulmonary illness. A trial was registered on 29 November 2021 to match the protection and immunogenicity of FAKHRAVAC and Sinopharm booster doses for adults who are vaccinated by Sinopharm.
A phase 2 trial was registered in December 2021 to gauge the protection and immunogenicity of SARS-COV-2 vaccine (Vero Cell) in adults who’ve obtained the two prime doses of one in all SARS-COV-2 Vaccines (Vero Cell inactivated-Sinopharm SARS-COV-2 Vaccine, CoronaVac SARSCOV-2 Vaccine, AstraZeneca SARS-COV-2 Vaccine, or Cominarty/Pfizer mRNA COVID-19 Vaccine). One other section 2 trial registered in December 2021 goals to gauge the immunity, security and reactogenicity of various SARS-CoV-2 vaccines specifically Sinopharm’s BIBP, CanSinoBIO and AstraZeneca ChAdOx.
-
JANSSEN/JOHNSON&JOHNSON (USA)
J&J has developed vaccines for Ebola and different ailments with Recombinant Adenovirus Serotype 26 (Ad26) and has now made one for the coronavirus. It launched section 1/2 trials in July 2020, and in September a section 3 trial with 60,000 contributors in Latin America. It hopes to make as much as a billion doses in 2021. Janssen started section 3 trials within the UK in November final 12 months. J&J has dedicated 500 million doses of this vaccine to the COVAX initiative for distribution worldwide.
In January, the corporation introduced that the vaccine had an efficacy of 66% in Latin America, 57% in South Africa and 72% in the US, with 100% efficacy towards extreme illness in all trials. In June 2021, the corporation launched a phase 4 trial within the Netherlands. J&J is among the many vaccines within the COVAX portfolio. It’s recognized that many individuals, together with a big variety of individuals with several sclerosis (PWMS), who take medicines that chronically suppress the immune system don’t produce neutralizing antibodies to COVID-19 proteins in response to vaccination. To check this, a phase 4 trial was registered on 18 October 2021 to see whether or not giving booster doses of the COVID-19 vaccine to individuals with several sclerosis can enhance the immune response to COVID-19. A phase 3 trial was registered on 25 October 2021.
A recent study report printed on 22 October 2021 proved that the J&J vaccine was efficient as a booster. A phase 1/2 trial registered in November 2021 goals to handle proof gaps concerning the protection, reactogenicity and immune responses of a heterologous enhancement of a single dose of J&J vaccine (half or full dose) in recipients who’ve obtained both 1-dose or 2-doses of Sinovac and/or Sinopharm’s inactivated COVID-19 vaccines. A phase 3 trial was begun in October 2021 to match brief and long-term immunogenicity of various COVID-19 vaccine combos towards the ancestral SARS-CoV-2 in addition to totally different variants of concern. Involving 600 contributors, the trial is because to be accomplished by April 2023.
-
MODERNA/NATIONAL INSTITUTE OF ALLERGIES AND INFECTIOUS DISEASES (US)
RNA VACCINE
In collaboration with the Nationwide Institute of Allergic Reactions and Infectious Ailments (NIAID), US-based Moderna initially launched a phase 1 trial to check their second COVID-19 vaccine, mRNA-1273.351. The vaccine particularly targets the SARS-CoV-2 Beta variant, which was first recognized within the Republic of South Africa. The mRNA-1273.351 vaccine candidate might be given to trial contributors in vaccination schedules alone, sequentially, or co-administered with Moderna’s first candidate, mRNA-1273, which has already been permitted and rolled out within the US. A phase 2 trial, which can test the efficacy of the vaccine towards COVID-19 in most cancer sufferers, was launched in April 2021 with 120 contributors.
In June, Moderna launched a phase 4 trial for mRNA-1273.351 in Belgium. On 11 August 2021, outcomes from a phase 2/3 trial instructed that the vaccine was secure to make use of and efficacious in stopping COVID-19 in adolescents between 12 and 17 years previous. On the same day, outcomes from a phase 3 trial of a third-dose vaccine in grownup transplant recipients confirmed that the booster enhanced their immune response. The USA Food and Drug Administration has authorised the use of booster shots in transplant recipients. A phase 4 trial was registered on 1 September 2021 to check the efficacy of booster vaccination in kidney transplant sufferers who didn’t seroconvert after two doses of vaccine. A phase 4 trial was registered on 12 October 2021 and expects to enrol 525 contributors. The research is anticipated to start in November 2021.
This trial goal is to measure the humoral and adaptive immune response in sufferers with most cancers’ prognosis present process mRNA vaccination towards SARS-CoV-2. On 14 October 2021, a phase 2 trial was registered with plans to enrol 400 contributors. The research aims to induce an enhanced antibody response to COVID-19 in kidney and liver transplant recipients who’ve destructive or indeterminate anti-spike antibodies after not less than two Moderna or Pfizer-BioNTech mRNA COVID-19 vaccines. Individuals might be randomized to obtain a Moderna or Pfizer-BioNTech COVID-19 booster dose or obtain the booster dose along with a short-lived, prescribed discount of their upkeep immunosuppression (IS) routine. In November 2021, the corporation registered a phase 2 study to gauge the immunogenicity and security of the corporate‘s mRNA-1283 COVID-19 vaccine boosters to spice up antibody responses to the ancestral pressure of SARS-CoV-2 virus, the B.1.351 variant, and probably different SARS-CoV-2 variants.
-
WUHAN INSTITUTE OF BIOLOGICAL PRODUCTS (CHINA)
INACTIVATED VACCINE
The United Arab Emirates became the primary international nation to approve this vaccine after interim section 3 trials confirmed 86% efficacy. The Wuhan Institute launched section 3 trials in July 2020 within the UAE, and in Morocco and Peru in August 2020. The state-owned Chinese language firm Sinopharm has been placing the vaccine using medical exams. On 14 Sept, the UAE gave emergency approval for the vaccine to be given to well-being care staff.



